Our understanding about the T-cell-mediated immune processes that underlie MS pathogenesis and regulation has improved considerably over the past several years. However, the laboratory tests available for MS do not utilize this advanced level of knowledge. In fact, the diagnosis of this disorder still depends on a conglomeration of clinical, laboratory and radiographic criteria that can be viewed as tests for a similar “end result” rather than a “biologic process”. Thus, there is considerable heterogeneity in the clinical picture as well as in individual responses to therapy. In diagnostically difficult cases or in patients that have exhibited only a single clinical episode, a definitive diagnosis may be considerably delayed. An early definitive diagnosis of MS is highly desirable because early treatment of this disease (starting when the patients have low disability scores) is more effective in the long term. Also, immunomodulatory therapies for MS are not benign and should be avoided without a definitive diagnosis.
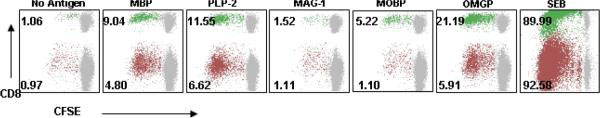
Currently, there is no immunologic test used for the diagnosis of MS, with the exception of oligoclonal bands and immunoglobulin (IgG) synthesis testing in cerebrospinal fluid (CSF). These CSF tests are neither sensitive nor specific indicators of MS. Furthermore, while several immune-based therapies are employed in the treatment of this disease, the monitoring of the immune system is not part of standard patient care, mainly due to the lack of robust, reproducible and clinically applicable assays. Thus, the development of an immunologic assay that can be performed by clinical laboratories, at the same time putting into application our current state of knowledge, would make a major impact on current management of MS. The main requirement of such an assay would be the ability to immunologically differentiate MS from other neurologic diseases (OND) and healthy subjects and to monitor this immunologic readout through the course of therapy. While the generation of such a test has been technologically difficult, we have recently adapted a flow cytometric proliferation assay that allows for the detection, quantification and phenotyping of CNS-reactive T-cell responses following a relatively short-term in vitro culture. We believe that simple modifications and adaptations of this system would be sufficient to develop a robust assay that can be used in clinical decision-making. We are actively pursuing the validation of such an assay system.
Learn More:
Multiparameter Flow Cytometric Assays to Quantify Effector and Regulatory T-Cell Function in Multiple Sclerosis. Sinha S, Crawford MP, Ortega SB, Karandikar NJ. J Mult Scler (Foster City). 2015 Jan;2(1). pii: 1000130. PMID: 26137595