The following two projects are led by Sterling B. Ortega, PhD, Associate of Pathology.
Project A
The role of T-cells in stroke recovery
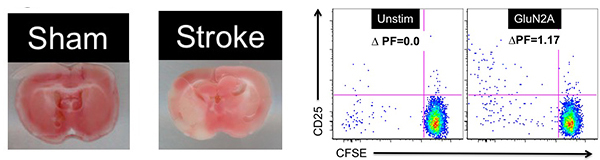
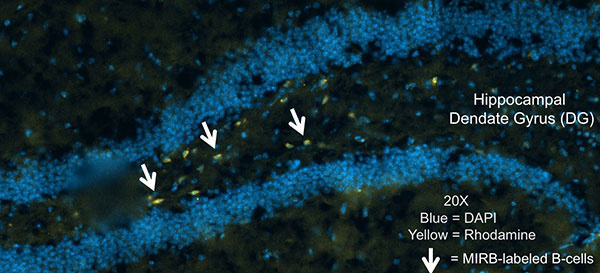
Stroke is caused by a loss of blood flow to the brain, in some cases resulting in permanent neurological damage. Unfortunately, there is no FDA-approved neurotherapeutic to reverse this damage and promote functional recovery. Our previous research has made significant advances in understanding the neuro-immunological interplay by demonstrating the possible opposing roles that immune cells play during stroke recovery. My previous research showed that B-cells ameliorate stroke severity, while novel neuronal-reactive CD8+ T-cells were robustly activated following an ischemic injury (the focus of this project). CD8 T-cells are potentially detrimental as their function includes cytotoxicity to target cells, and in the case of stroke, the very cells which are undergoing repair. Our preliminary data show that CD8 T-cells are reactive to a neuronal peptide in multiple mouse strains. These CD8 T-cells are inflammatory, and infiltration into the CNS by these CD8 T-cells correlates with a decrease in motor function recovery. In fact, in a pediatric patient cohort, we observed CD8 T-cell reactive to brain antigens only in patients with heart/lung treatment which subsequently developed brain injury.
The proposed project will investigate the role of stroke-induced GluN2A (neuronal)-specific CD8+ T-cells as secondary mediators of neuronal cell death following an ischemic injury, thus inhibiting endogenous motor function recovery. We will characterize these cells, and determine if they can indeed directly cause neuronal cell death. We will also determine if these cells can cause motor/behavior dysfunction in vivo, a phenomenon that is not directly dependent on neuronal killing.
In summary, we will delineate the cellular targets, mechanisms, and motor/behavior dysfunction stroke-induced neuronal-reactive CD8 T-cells possess using in vitro and in vivo approaches. Investigating the immune mechanisms that may potentiate an ischemia-induced brain injury is a novel approach to understanding how the immune system can be targeted in order to maximize recovery from a neurovascular disease that affects millions of people each year.
Learn More:
Stroke induces a rapid adaptive autoimmune response to novel neuronal antigens. Ortega SB. Noorbhai I. Poinsatte K. Kong X. Anderson A, Monson NL, Stowe AM Discov Med 2015; 19(106):381-92. PMID: 26105701
Preconditioning-induced CXCL12 upregulation minimizes leukocyte infiltration after stroke in ischemia-tolerant mice.Selvaraj UM, Ortega SB, Hu R, Gilchrist R, Kong X, Partin A, Plautz EJ, Klein RS, Gidday JM, Stowe AM. J Cereb Blood Flow Metab 2017 Mar;37(3):801-813. PMID: 27006446
Repetitive hypoxic preconditioning modulates B-cells during endogenous protection in murine stroke. Monson NL, Ortega SB, Ireland SJ, Meeuwissen AJM, Chen D, Plautz E, Shubel E, Kong X, Li MK, Freriks LH, Stowe AM.J Neuroinflammation. 2014 Jan 31;11:22. PMID: 24485041
Project B
Brain-targeting adaptive immunity in pediatric Extracorporeal Membrane Oxygenation (ECMO) patients with acquired brain injury
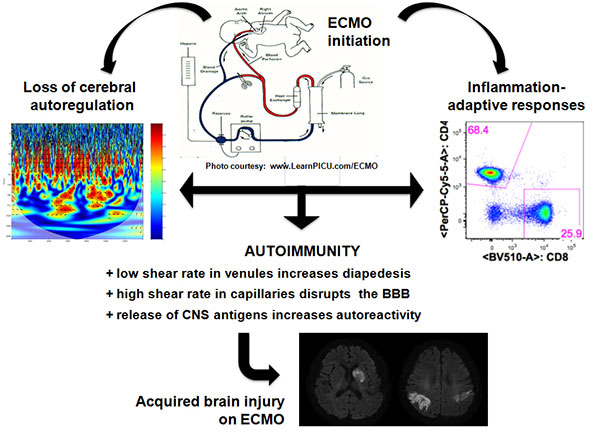
The long-term goal of this research project is to develop a therapy for ECMO-mediated neuropathology. ECMO is a technique used to provide circulation and gas exchange during acute cardiac and/or pulmonary failure in young children with respiratory failure, congenital heart disease, and sepsis. As the usage of ECMO treatment increases so has the occurrence of systemic inflammatory response syndrome and neurological injury in pediatric patients. Acquired neurological injury is a significant risk factor and contributes to morbidity and mortality in patients on ECMO, with complications secondary to ECMO including intracranial hemorrhage, cerebral infarction, and seizures. While neurological injury may be secondary to detrimental mechanisms associated with the underlying disease, our recent preliminary data suggest that inflammation may exacerbate co-morbidities in these patients.
ECMO increases the production of many inflammatory cytokines, such as IL1β, IL6, IL8, and TNF-α within hours of initiation. Animal studies show increased inflammatory cytokines in many tissues, including the brain. A combination of innate inflammation and loss of cerebral blood pressure autoregulation could result in blood-brain barrier disruption allowing for penetration of innate cells and the presentation of central nervous system antigens to the adaptive immune system leading to an induction of inflammatory CNS-targeting adaptive immune response. In fact, the very characteristics (accessibility, specificity, and memory) that define the adaptive immune system is what makes these cells potent harbingers of neuropathology.
Accessibility, in that these cells interrogate all cells of the body in order to maintain health. Specificity, in that once these cells are presented with their cognate antigen - any future encounter with the same antigen will elicit a response. Finally, memory, in that a minor population of these activated cells will go on to become long-lived memory cells which can continue to affect their function for years.
Our preliminary data (In Press) shows that in pediatric patients, the ECMO procedure induces a robust inflammatory response (IL6, IL8) that is detectable in the plasma of ECMO patients. This inflammation is robust enough to drive healthy adaptive immune cells to an inflammatory phenotype (lL17 and IFN-γ). Secondly, CNS injury coincides with activated (CD25+) adaptive (T and B-cells) immune cells. Interestingly, we have detected T-cell responses toward to brain antigens in ECMO patients that subsequently developed white matter tissue injury. Based on our preliminary data, we will test the hypothesize that ECMO induces neuropathology mediating adaptive immune responses.
Learn More:
A pilot study identifying brain-targeting adaptive immunity in pediatric Extracorporeal Membrane Oxygenation (ECMO) patients with acquired brain injury. Ortega SB, Pandiyan P, Windsor J, Torres VO, Selvaraj UM, Lee A, Morriss M, Tian F, Raman L, Stowe AM. In Press
