A. The function of SIRPγ in human T-cells
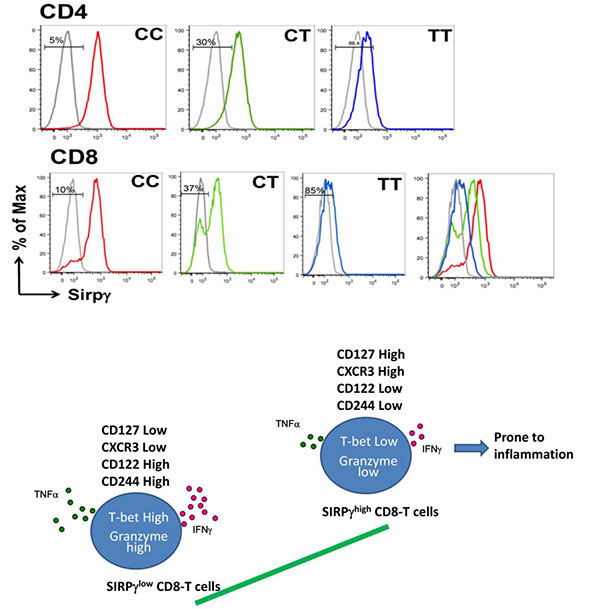
Signal regulatory protein g (SIRPγ) is uniquely expressed by human T-cells, compared to other immunomodulatory SIRP family members. The role of SIRPγ in T cell function is largely uncharacterized despite accumulating evidence in the literature linking SIRPγ to autoimmune diseases including T1D and SLE. To undertake this critical task of investigating it directly in the human immune system, we have developed two powerful approaches 1) identification of healthy individuals harboring T-cells with genetically coded “abnormal” (in this case reduced) SIRPγ expression; 2) knockdown of SIRPγ in primary human T-cells. We discovered that a single nucleotide polymorphism causing a C/T variant in SIRPγ, rs2281808, correlated with SIRPγ expression on T-cells (manuscript in minor revision). Rs2281808 CC genotype was associated with robust SIRPγ expression on the majority of CD4 and CD8 T-cells. In contrast, CD4 and CD8 T-cells from TT carriers had significantly reduced surface expression of SIRPγ, whereas the CT genotype was associated with intermediate SIRPγ expression. We have used this approach to show that the level of SIRPγ expression compartmentalized CD8 T-cells into strikingly distinct phenotypic and functional populations. Reduction of SIRPγ on CD8-T cells was associated with heightened effector state and increased expression of genes and molecules associated with migratory and cytotoxic potential. Likewise, SIRPγlow CD4 T-cells produce significantly more IFNγ and perforin than their SIRPγhigh counterparts. SIRPγ low CD8 T-cells were also enriched for transcription factors that are required to maintain effector T-cells identities. Collectively, these findings indicate that SIRPγ might be an important checkpoint regulator of human effector T-cells. Importantly, SIRPγ knockdown in primary human T-cells caused increased secretion of IFNγ, TNFα and granzyme B from CD8 T-cells and IFNγ from CD4 T-cells upon activation, thus providing direct evidence that reduced SIRPγ expression can potentiating effector responses in human T-cells. In line with these findings, SIRPγ low CD8 T-cells are enriched within canonically defined terminal effectors regardless of genotype. Interestingly rs2281808 genotype altered distribution of SIRPγ low cells: CT and TT, but not CC, also have enrichment of these cells within the naïve and central memory T-cells. Collectively our preliminary studies raise three exciting possibilities that we are currently exploring in the lab 1) reduced SIRPγ expression leads to exaggerated effector responses from human T-cells; 2) human T cells downregulate SIRPγ to differentiate into terminal effectors; 3) SIRPγ low T-cells are imprinted to function as terminal effectors, irrespective of their (canonically defined) differentiation status.
B. The functional implications of rs2281808 T variant and Sirpγlow expression on T cells in the context of autoimmunity
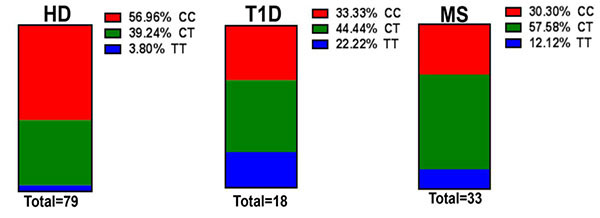
There has been accumulating evidence in the literature connecting SIRPγ with autoimmune diseases. Multiple GWAS studies have shown that the SNP rs2281808 C/T variant, present within the Signal Regulatory Protein Gamma (SIRPG) gene, is associated with type 1 diabetes (T1D). Interestingly, early onset T1D patients provided more association evidence for rs2281808. Further, we have recently found the T variants (CT and TT) of this SNP at a higher frequency in relapsing-remitting multiple sclerosis (RRMS). Differential expression of SIRPγ has also been reported in SLE, suggesting SIRPγ may play a key role in immune dysregulation in multiple different autoimmune diseases. Since T-cells are critical pathogenic players in both T1D and RRMS and SIRPγ is expressed by human T-cells, we reason that SIRPγ might be regulating pathogenic responses in human T-cells. Our long-term goal is to identify the mechanisms by which the rs2281808 T variant in SIRP gene predisposes/potentiates autoimmunity.
Collectively, our studies will provide fundamental insights into SIRPγ-mediated regulation of immune responses in human CD4 and CD8 T-cells. Understanding the role of SIRPγ in immune regulation will provide a framework for future studies to define the role of SIRPγ in autoimmunity, and may have a broad impact by enlightening our understanding of manipulating SIRPγ to enhance immune response for the treatment of infections and cancer. Further our studies will determine whether SIRPγ can be a functional marker for identifying T-cells with terminal effector functions. If so, this would be a powerful new prognostic and diagnostic tool.
Learn More:
An autoimmune disease risk SNP, rs2281808, in SIRPG is associated with reduced expression of SIRPγ and heightened effector state in human CD8 T-cells. Sinha S, Borcherding N, Renavikar PS, Crawford MP, Tsalikian E, Tansey M, Shivapour ET, Bittner F, Kamholz J, Olalde H, Gibson E, Karandikar NJ. Sci Rep. 2018 Oct 18;8(1):15440. doi: 10.1038/s41598-018-33901-1. PMID: 30337675